Blood pressure and heart condition meds recalled. Pills were put in the wrong bottles
Bottles of medication designed to keep blood pressure down contained medication designed to help prevent strokes and blood clots in people with heart conditions, causing a recall of one lot of each.
This packaging mixup comes from Golden State Medical Supply, which said in its FDA-posted recall notice that it learned that a bottle labeled as having 25 mg tablets of hypertension drug Atenolol actually had 75 mg tablets of Clopidogrel. So, Golden State yanked lot No. GS046745 of Atenolol and Clopidogrel.
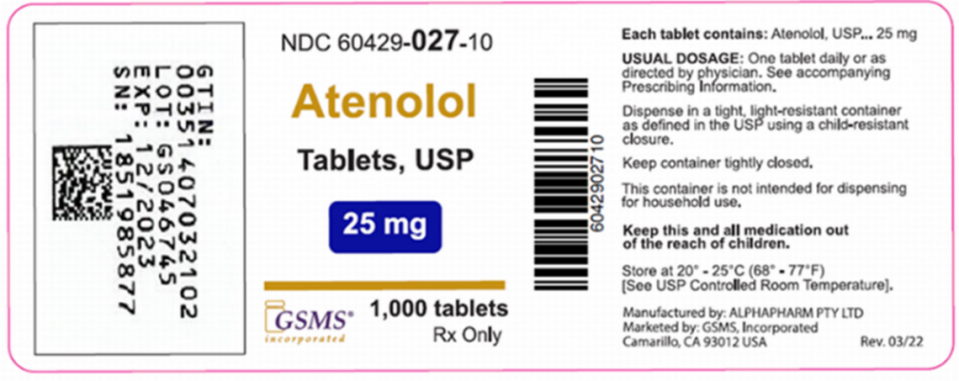


Patients and caregivers should take the recalled drugs back to their pharmacy or contact the prescribing medical professional for instructions on what to do.
Consumers and distributors with questions about this recall can contact Golden State at 800-284-8633, ext. 116, 10:30 a.m. to 7 p.m., Eastern time.
If these or any drugs cause a problem, after notifying a medical professional let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Then, notify the manufacturer.

