Introducing the Nuclear Periodic Table of Elements
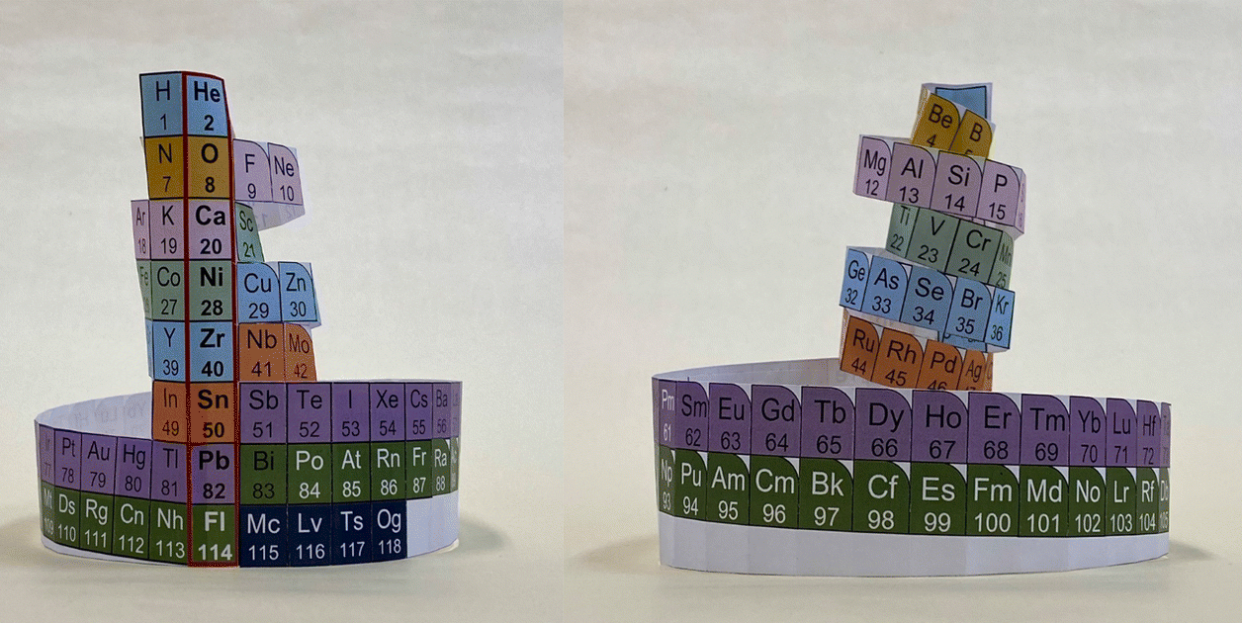
A new version of the periodic table arranges elements by protons instead of electrons.
The perennially useful original Mendeleev periodic table has led to spinoffs, including for quantum dots.
More stable nuclei are in the center, and they grow more deformed as you move outward.
Japanese scientists have made a new (nu?) periodic table organized by the number of protons in the nucleus instead of the element’s number of electrons. They call it the Nucletouch table, and where the existing table gathers around key numbers for electrons, the Nucletouch table does the same for protons.
In a new paper in Foundations of Chemistry, Kyoto University's Yoshiteru Maeno and Kouichi Hagino explain their rationale in detail:
“Atomic nuclei are located at the center of atoms and carry almost all the fraction of the mass of atoms. They consist of a small number of protons and neutrons, collectively called nucleons. In this picture, nucleons occupy single-particle orbits, like electrons in atoms, which naturally leads to the concept of shell structure and shell closures.”
The classic Mendeleev periodic table sorts elements by number of electrons and then in groups that indicate how readily their atoms bond with others. That bondability factor is based on shell structure and shell closure. For the Nucletouch table, Maeno and Hagino focused on the way nuclear protons arrange themselves into similar patterns within the nucleus.
“Such shell structure in atomic nuclei have been evidenced by many phenomena, such as an increased binding energy, discontinuities of the nuclear radius as well as neutron and proton separation energies (corresponding to the ionization energy in atoms), and an increased excitation energy of the first excited state, all of which occur at the shell closures,” they write. So the shape, bondability, and other factors of the nucleus are influenced by whether or not the shell structure is filled and stable.
For electrons, the shell levels are full at 2 in the first, 8 in the second, and so forth. Overall, the numbers representing full-up electron shells are 2, 10, 18, 36, 54, and 86. For nucleons, including protons, the numbers are 2, 8, 20, 28, 50, 82, and 126. These values are called magic numbers.
“Thus, tin (atomic number 50), with 50 protons in its nucleus, has 10 stable isotopes, whereas indium (atomic number 49) and antimony (atomic number 51) have only 2 stable isotopes apiece,” Britannica explains.
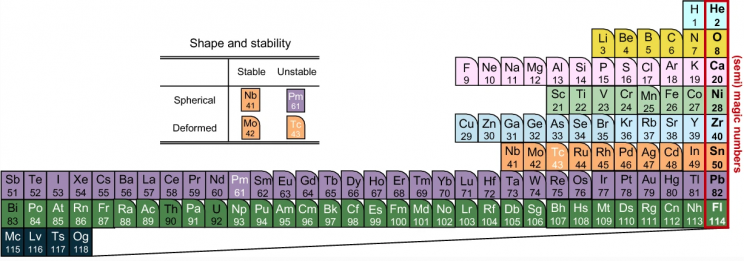
Maeno and Hagino centered stable nuclei in their Nucletouch table. “In our nuclear periodic table, we also see that nuclei tend to be spherically-shaped near the magic numbers, but deformed as you move away from them,” Hagino said.
Overall, the scientists hope the table will give people a new way to examine the elements at a glance, including a new way to identify similarities that could benefit chemists and physicists.
“The nuclei in the vicinity of the shell closures can be interpreted as one or two protons or proton holes outside the shell closures, and thus may have similar properties to one another,” they write. In many experimental processes, trying multiple chemically similar, but different atoms could lead to more practical designs. Scientists can be surprised by elements that perform better, cost less, and so forth.
And, they add, “It has been known that some of the nuclear magic numbers are changed in neutron-rich nuclei.” This means isotopes with an especially plentiful supply of neutrons in proportion to their electrons, including radioactive isotopes. “It might be amusing to construct an extended version of nuclear periodic table by taking into account such shell evolution in exotic nuclei.”
You Might Also Like


